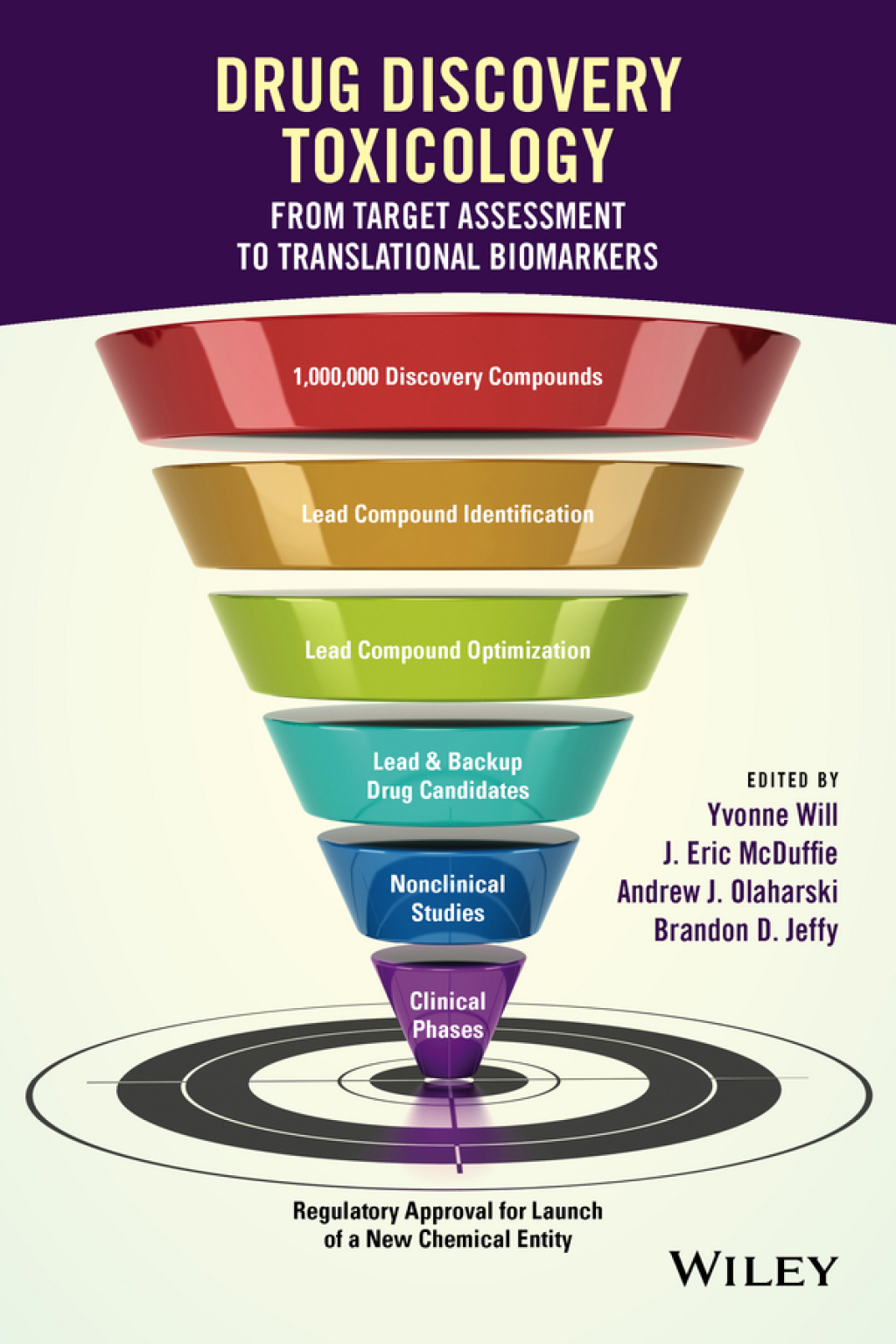
As a guide for pharmaceutical professionals to the issues and practices of drug discovery toxicology, this book integrates and reviews the strategy and application of tools and methods at each step of the drug discovery process. • Guides researchers as to what drug safety experiments are both practical and useful • Covers a variety of key topics – safety lead optimization, in vitro-in vivo translation, organ toxicology, ADME, animal models, biomarkers, and –omics tools • Describes what experiments are possible and useful and offers a view into the future, indicating key areas to watch for new predictive methods • Features contributions from firsthand industry experience, giving readers insight into the strategy and execution of predictive toxicology practices
“Advances in Anesthesia, 2022 1st Edition” has been added to your cart. View cart
Drug Discovery Toxicology: From Target Assessment to Translational Biomarkers From Target Assessment to Translational Biomarkers 1st Edition
Author(s): Yvonne Will; J. Eric McDuffie; Andrew J. Olaharski; Brandon D. Jeffy
Publisher: Wiley-Blackwell
ISBN: 9781119053330
Edition: 1st Edition
$39,99
Delivery: This can be downloaded Immediately after purchasing.
Version: Only PDF Version.
Compatible Devices: Can be read on any device (Kindle, NOOK, Android/IOS devices, Windows, MAC)
Quality: High Quality. No missing contents. Printable
Recommended Software: Check here